Protein Structure: Difference between revisions
| Line 78: | Line 78: | ||
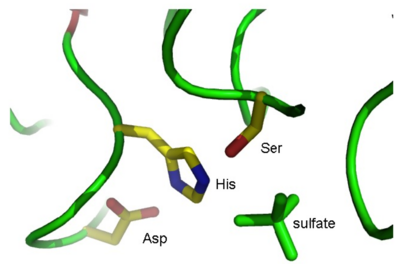
[[Image:RGAE_active_site.png|thumb|center|border|400px|'''Figure 3.''' The active site in RGAE.]] | [[Image:RGAE_active_site.png|thumb|center|border|400px|'''Figure 3.''' The active site in RGAE.]] | ||
Let’s try to look at the surface of the interaction now. You can create an object called “antibody”, that contains both antibody chains (chain H+L) by the command select, and show as surface. | Let’s try to look at the surface of the interaction now. You can create an object called “antibody”, that contains both antibody chains (chain H+L) by the command select, and show as surface. | ||
'''Question 3.''' Provide a screenshot of the interaction surface. Which secondary structures of the allergen Is the antibody interacting with: alpha helices, beta-sheets or loops? | '''Question 3.''' Provide a screenshot of the interaction surface. Which secondary structures of the allergen Is the antibody interacting with: alpha helices, beta-sheets or loops? | ||
Now we want to colour which specific residues of the allergen are in close contact with the antibody (exactly at a distance of 4Å). We will call this object the “epitope”. To define the distance, we can use the command ''around''. | Now we want to colour which specific residues of the allergen are in close contact with the antibody (exactly at a distance of 4Å). We will call this object the “epitope”. To define the distance, we can use the command ''around''. | ||
In order to select the full residues and not just the atoms of the allergen that are in direct contact we have to do a trick and select by residue using the command ''byres''. | In order to select the full residues and not just the atoms of the allergen that are in direct contact we have to do a trick and select by residue using the command ''byres''. | ||
'''Hint:''' ''byres''.(XX ''around'' 6) if we want to select all residues in a distance of 6Å to XX | '''Hint:''' ''byres''.(XX ''around'' 6) if we want to select all residues in a distance of 6Å to XX | ||
Revision as of 20:16, 6 October 2025
By Carolina Barra Quaglia
Overview
In this exercise you will learn how to
- Search diferent protein databases to obtain protein structures.
- Critically choose the best structure, when more than one is available.
- Visualize a protein structure using PyMOL
- Highlight features of interest and perform a basic alignment.
This exercise is written a bit different. Instead of the step wise exercise we are going to try a different method called inquiry-based learning, where students explore real-world problems and questions, taking ownership of their learning by asking questions, conducting research, and developing their own solutions and understandings.
A bit of background
The question of today’s exercise is about allergens.
Allergens are fascinating because they aren’t random proteins; they cluster into certain protein families that repeatedly trigger allergic sensitization in humans.
However, one very interesting allergen protein family is the profilins. Here’s why:
They are highly conserved across species.
Profilins are small actin-binding proteins found in almost all eukaryotic cells (plants, animals, fungi).
Despite their ubiquity and structural conservation, humans typically only become allergic to plant profilins (e.g., from birch pollen, grass pollen, the rubber tree, or certain fruits/vegetables).
Today we would seek to understand:
Why only plant profilins are giving an allergenic response despite their high conservation across species?
Getting started
We know that protein functions are closely correlated to their 3D structure, so in order to understand what is different in Profilin proteins from plants and mammals we are going to gather some experimentally determined protein structures from plant and mammal proteins.
First go to the Protein Data Bank (PDB) https://www.rcsb.org/
We are looking for and antibody of the class E (those are related to allergy) in complex with a plant allergen (in this case we will use Hevea brasiliensis, the rubber tree).
And search for IgE AND Hev b 8
We are looking for a protein structure complex, so dismiss all the search results that do not contain a protein complex including both the IgE AND Hev b 8.
Question 1. Look at the method X-RAY diffraction (Å). Which of the complexes is of better quality and why?
Visualization & PyMOL
You can visualize the structure directly at the PDB website using a browser-based viewer (buttons are found below the structure image), but we will use the viewer PyMOL for our purposes. It is an excellent viewer that can also be used to prepare publication-quality images of protein structures, and it is a very valuable tool when working with protein structures.
⇒ If you have not already, download PyMol from the web site and install it on your computer.
The program has three panels:
- The viewer panel where the molecule will be displayed,
- an "objects panel" on the right, with a list of all your objects with the pull-down menus to show (S) or hide (H) elements,
- and the command-line panel in the bottom, where you can type commands.
⇒ Now select the best complex and load it into PyMOL using the fetch command.
(If you cannot solve question 1 use this alternate code to continue the exercise: fetch 7SBG)
You can toggle the object on and off by clicking on its name. Try this. To the right of the object name, there are five buttons: A(ction), S(how), H(ide), L(abel) and C(olor).
⇒ Remove the water molecules and cofactors (hetatm) used for making the crystal using the command remove (these are not part of the of the protein complex).
If you show the sequence, you will notice that each of the chains have a name H and L for the antibody (heavy and light chain) and C for the allergen.
⇒ Colour the different chains of the antibody (IgE) in yellow and blue and the allergen (Hev b 8) in green.
Question 2. Provide a screenshot of the complex.
Let’s try to look at the surface of the interaction now. You can create an object called “antibody”, that contains both antibody chains (chain H+L) by the command select, and show as surface.
Question 3. Provide a screenshot of the interaction surface. Which secondary structures of the allergen Is the antibody interacting with: alpha helices, beta-sheets or loops?
Now we want to colour which specific residues of the allergen are in close contact with the antibody (exactly at a distance of 4Å). We will call this object the “epitope”. To define the distance, we can use the command around.
In order to select the full residues and not just the atoms of the allergen that are in direct contact we have to do a trick and select by residue using the command byres.
Hint: byres.(XX around 6) if we want to select all residues in a distance of 6Å to XX
Question 4. Now colour the epitope in red and provide a screenshot. How many residues can you count that are in close contact with the IgE antibody?
Structure comparisons
Proteins exhibiting the same fold may occasionally have similar function, especially in the case of enzymes. However, when the proteins have reached the same fold by convergent evolution (or diverged a very long time ago), such similarities are not always obvious from sequence comparisons alone. Here, we will compare RGAE with platelet-activating factor acetylhydrolase (PAFA) from domestic cow (Bos taurus) and have a look at their active sites. These two enzymes have similar hydrolytic functions and catalytic residues but have no obvious sequence similarity (advanced alignment tools will identify approximately 20% identical residues).
⇒ First, fetch the structure for PAFA called 1WAB and open it in PyMOL along with the structure of RGAE. You will notice that the two structures are not aligned. To fix this, type the following:
align 1WAB, 1K7C
This will align the structure of PAFA (1WAB) with that of RGAE by moving the former. Navigate to the active site of RGAE found previously and identify the residues in PAFA corresponding to the active site residues in RGAE.
Q7 The active site residues of PAFA are: Ser_____, His_____ and Asp_____. Does this correspond to the residue numbering in RGAE? Why/why not?
Hint:
- if you color the active site amino acids in RGAE (1K7C) to something that is easy to recognize, it will be much easier to spot the overlap.
- Sequence viewer mode will also be a big help here - when you click a AA side chain in the other structure, the corresponding position will light up in the sequence view.
PyMOL links
- PyMOL home: http://www.pymol.org
- PyMOL manual: http://pymol.sourceforge.net/newman/userman.pdf
- PyMOL Wiki: http://www.pymolwiki.org/index.php/Main_Page
- PyMOL settings (documented): http://pymolwiki.org/index.php/Settings